11b-Hydroxysteroid dehydrogenase 1 (11b-HSD1) is a multi-functional carbonyl reductase

Figure 1. Scheme of some 11b-HSD1 substrates. G6P, glucose-6-phosphate; 6PG, 6-phosphogluconate.
Besides its well accepted role in the fine-tuning of glucocorticoid action, several studies addressed the substrate promiscuity of 11b-HSD1 and identified other oxo-steroid and sterol substrates, oxo-bile acids and xenobiotics (Figure 1, reviewed in Odermatt and Klusonova, 2015, J. Steroid. Biochem. Mol. Biol.).
Rotational symmetry between cortisone and 7-ketocholesterol bound to 11b-HSD1.

Figure 2. Rotational symmetry between cortisone and 7-ketocholesterol bound to 11b-HSD1. The rotational symmetry of steroids has nicely been described by Lathe R., 2002, Steroids, p967-977.
In an earlier study, we showed that human 11b-HSD1 efficiently converts 7-oxocholesterol (7-ketocholesterol) to 7b-hydroxycholesterol and provided evidence in a rat study that the rapid hepatic metabolism of oxidized cholesterol is relevant in vivo (Schweizer et al., 2004, J Biol Chem). Later, we showed a role of 11b-HSD1 in the metabolism of 7-oxolithocholic acid (7-oxoLCA) (Odermatt et al., 2011, Biochem J) and reported on the impact of 11b-HSD1 on bile acid homeostasis in mice (Penno et al., 2014). The role of 11b-HSD1 and of its cofactor supplier hexose-6-phosphate dehydrogenase (H6PDH) in oxysterol and bile acid homeostasis is currently further investigated (SNF project No. 31003A-159454). Also, the potential use of 7-oxoLCA as a functional biomarker of 11b-HSD1 activity is further investigated in this project.
That 11b-HSD1 can accept steroid substrates with 11-oxy as well as 7-oxy groups can be explained by the rotational symmetry of these molecules (Figure 2).
Formation of CDCA and UDCA from supplied 7-oxoLCA by 11b-HSD1 from different species
Figure 3. Formation of CDCA and UDCA from supplied 7-oxoLCA by 11b-HSD1 from different species.
11b-HSD1 is a good example to demonstrate species-specific differences. This is crucial when choosing an animal model and when trying to extrapolate results from an animal model to human. Rodents often are the preferred species for preclinical investigations. While rat and mouse 11b-HSD1 efficiently metabolize 7-oxocholesterol and 7-oxoLCA, like their human counterpart, guinea-pig 11b-HSD1 showed very weak activity towards 7-oxocholesterol and 7-oxoLCA. The latter is a major bile acid in guinea-pig but only a minor metabolite in human. Moreover, human 11b-HSD1 formed predominately CDCA from 7-oxoLCA, whereas the rat and mouse enzymes formed almost equal amounts of CDCA and UDCA (Figure 3).
Species differences in the oxoreduction of triadimefone by 11b-HSD1

Figure 4. Species differences in the oxoreduction of triadimefone by 11b-HSD1. T0505, selective 11b-HSD1 inhibitor. KO, microsomes from 11b-HSD1 knockout mice. Triadimefone and triadimenol were measured by UPLC-MS/MS.
Additionally, 11b-HSD1 has a role in phase I biotransformation reactions and catalyzes the carbonyl reduction of several non-steroidal xenobiotics.
Rat and mouse 11b-HSD1 are much less efficient in catalyzing the oxoreduction of triadimefone and bupropion than the human enzyme (Figure 4.), revealing important species differences.
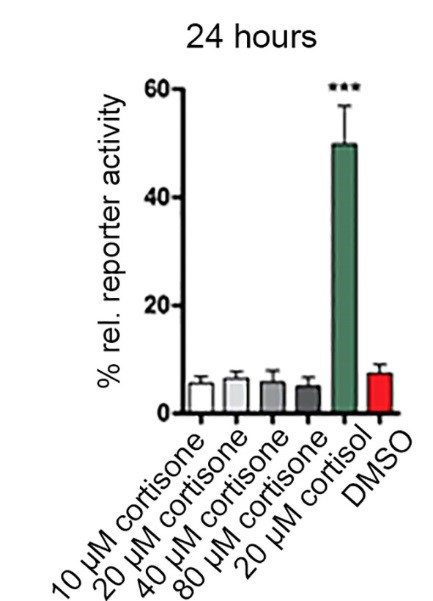
Figure 5. GRIZLY assay for the evaluation of GRE activation by glucocorticoids in zebrafish larvae. GRE:luc larvae (n = 48) were treated with cortisone or cortisol, and the bioluminescence response was monitored over time. DMSO served as vehicle control.
Species differences need also to be considered when developing inhibitors and in the interpretation of results from preclinical studies. Comparison of several inhibitors on human, hamster, mouse, rat, guinea-pig and dog 11b-HSD1 revealed large differences in the respective inhibitory potency (Arampatzis et al., 2005, J Mol Endocrinol). Similarly, we observed significant species differences in the inhibition of 11b-HSD2 by the anabolic androgenic steroid fluoxymesterone (Fürstenberger et al., 2012, Toxicol Sci) and by the azole fungicides itraconazole and posaconazole (Beck et al., 2017, Biochem Pharmacol).
Lately, zebrafish gained increased attention as a model organism for toxicological studies but also to investigate endocrine effects. Regarding glucocorticoid metabolism, we recently reported the absence of 11b-HSD1 in zebrafish (Tsachaki et al., 2017, J Endocrinol). Thus, 11-ketoglucocorticoids such as cortisone and prednisone cannot be converted to the respective 11b-hydroxy form and are inactive in this species (Figure 5). Such species differences are important to know for appropriate use as model to study effects of xenobiotics on endocrine functions.
Identification of substrates for “orphan” SDRs and characterizing their functions

Figure 6. Rotational symmetry of 5α-DHT and cortisone. 3D structures of cortisone and 5α-DHT are shown in the upper panel. Cortisone rotated 180° (East-West rotation) is shown in the lower panel on the left, and overlay of 5α-DHT and the East-West rotated cortisone, revealing the close proximity of the 3-oxo group on 5α-DHT and the 20-oxo group on cortisone, is shown on the lower panel on the right (Araya et al., 2017, J Steroid Biochem Mol Biol).
Among the 75 SDR members in human, approximately 50% of the enzymes are not or only poorly characterized. Using our knowledge in ligand-protein interactions, we attempt to identify substrates of such “orphan” SDRs. We recently provided evidence for a potential role of DHRS7 as a tumor suppressor in prostate cancer (Seibert et al., 2015, Cancer Med). As the closest relative of DHRS7 is 11b-HSD1, we tested several steroids, sterols and xenobiotics as potential substrates of DHRS7. 5a-Dihydrotestosterone (DHT) was found to be a substrate of DHRS7 (Araya et al., 2017, J Steroid Biochem Mol Biol). Whether this is of physiological relevance is currently investigated (SNF project No. 31003A-159454). Additionally, weak activity towards cortisone was detected, indicating that DHRS7 possesses 3a/20b-hydroxysteroid dehydrogenase activity. Again, the rotational symmetry of the steroid molecules provides an explanation for this promiscuity of substrate recognition by DHRS7 (Fig. 6).
Prof. V. Wsol and coworkers at Prague, Czech Republic, reported quinone compounds and xenobiotics as possible substrates of DHRS7 (Stambergova et al., 2016, J Steroid Biochem Mol Biol), thus resembling 11b-HSD1 in its substrate promiscuity. Together, these results provide a basis to uncover the physiological function of DHRS7 and its potential role as tumor suppressor.
Identification of SDR inhibitors
Several SDRs are of potential interest for the development of therapeutic inhibitors. In order to identify novel classes of inhibitors against specific SDRs, we follow an approach involving structural modeling, compound library screening, bioassays and in vitro functional verification of enzyme activities. In collaboration with PD Dr. D. Schuster, University of Innsbruck, we applied pharmacophore models of 11b-HSD1, 17b-HSD1, 17b-HSD2, and 17b-HSD3 to screen chemical databases, and successfully identified novel inhibitor classes for these enzymes in several studies (reviewed in Beck et al., 2017, J Steroid Biochem Mol Biol; Kaserer and Beck et al., 2015, Molecules). This strategy of molecular modeling, in silico screening of the human metabolome database, and biologic evaluation of the hits, is now also applied in an attempt to identify additional substrates of SDRs.
