Leader: Dr. Markus L. Lampert
Post-Doc: Dr. Fabienne Böni
Introduction
Health-care systems are typically divided in different sectors providing different levels of care. Patients particularly those with chronic conditions move between these sectors. In order to create a continuum of care, the episodes should be linked. Such integrated care, however, is difficult to achieve in daily practice. Disruption and fragmentation are often the reality and cause adverse health outcomes.
At transitions from one sector to another, therapy changes may be overlooked or not implemented due to poor communication between health-care providers and insufficient knowledge of the patient. Unintended medication discrepancies may result which – in consequence – increase the risk of adverse drug events causing unplanned health resource utilization including early hospital readmission.
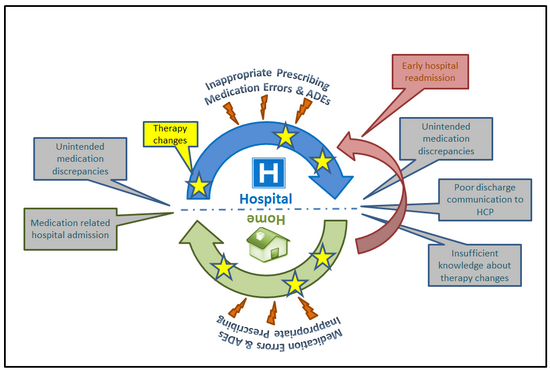
«Worst case scenario» depicting drug-related problems with a focus on the interface between different levels of health care (ADE Adverse drug event; HCP Health care professional).
Our vision
To improve the situation, research is needed to evaluate quantitatively and qualitatively the existing problem and to investigate the effects of improvement measures. But also, development is needed to create and validate instruments that help identifying patients at risk or facilitate and support the process of transitions. For this purpose, we developed a framework for research and development, called MOSAIC: Medicines Management Optimisation by structured assessment in integrated care.
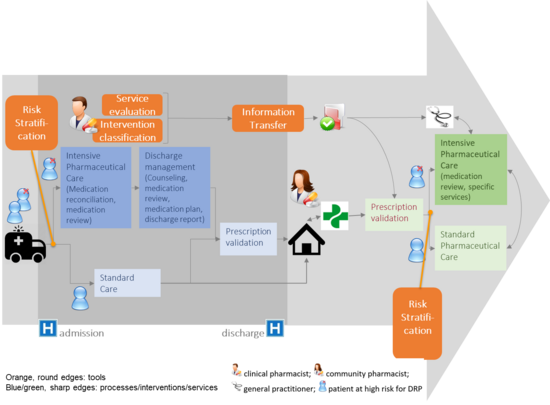
Project: Risk Stratification
Within the MOSAIC framework, an important aspect is the possibility to stratify patients according to their risk of developing a significant drug related problem. In order to perform such a risk stratification, instruments are needed which are easy to apply in daily routine and are of appropriate sensitivity and specificity. Identification of high-risk patients would allow to allocate the workforce of clinical pharmacist and other health-care professionals to those who are most in need of intensified pharmaceutical care. The development of such a risk stratification tool is presented in this work: the DART (Drug associated risk tool) is a self-assessment questionnaire with 34 items addressing risk factors for drug-related problems. A high specificity and sensitivity (88% resp. 67%) allow a reliable detection of existing risk factors.
PhD students
2010-2015: Carole Kaufmann: „Identification of Risks for Drug-Related Problems”
2014-2018: Dominik Stämpfli: „Drug associated risk tool (DART): score development and implementation into clinical practice
DART
DART: Probandenfragebogen (Deutsch)
DART: Erläuterungen/ Manual (English)
Artikel
Kaufmann CP, Stämpfli D, Hersberger KE, et al. Determination of risk factors for drug-related problems: a multidisciplinary triangulation process. BMJ Open 2015;5(3):e006376 Publication
Kaufmann CP, Stämpfli D, Mory N, et al. Drug-Associated Risk Tool: development and validation of a self-assessment questionnaire to screen for hospitalised patients at risk for drug-related problems. BMJ Open 2018;8(3) Publicatio
Stämpfli D, Boeni F, Gerber A, et al. Assessing the ability of the Drug-Associated Risk Tool (DART) questionnaire to stratify hospitalised older patients according to their risk of drug-related problems: a cross-sectional validation study. BMJ Open 2018;8:e021284 Publication
Project: Documentation and Communication
Since communication between the different settings is crucial to achieve continuity of care, the second project aimed at developing standards for classification of pharmaceutical interventions using a common terminology along the patient’s pathway.
The PharmDISC (Pharmacist’s documentation of interventions in seamless care) classification resulted from a development process using a mixed methods approach. The classification is validated in terms of feasibility, acceptability, appropriateness, and interpretability and reaches substantial interrater reliability (Fleiss’ Kappa 0.61)
PhD student
2013-2016: Karen Maes: „PharmDISC: a new system to document pharmaceutical interventions“
PharmDISC
PharmDisc - Klassifizierung von pharmazeutischen Interventionen Deutsch
Deskriptives Manual - Anleitung zum Pharm-DISC Klassifikationssystem für die Dokumentation pharmazeutischer Interventionen mit BeispielenDeutsch
PharmDISC - Classification d'interventions pharmaceutiques Français
Manuel descriptif - Instruction du système de classification PharmDISC pour la documentation des interventions pharmaceutiques avec explications et exemples Français
Project : KIRSCH Leitlinie «Konsequente Implementierung eines phaRmazeutischen Medikationsmanagements zur Erhöhung der Sicherheit an der SCHnittstelle Spitalaustritt» aus. Link
PhD Students
since 2016: Helene Studer
2016-2020: Tamara Imfeld-Isenegger
![[Translate to English:] Markus Lampert](https://pharma.unibas.ch/fileadmin/_processed_/5/4/csm_markus_lampert_a0bd5c951c.jpg)
Dr. Markus Lampert
Pharmaceutical Care
Petersplatz 14, Postfach 2148
4001 Basel
Switzerland
Tel: +41 62 311 52 30
