Functionalized Calcium Carbonate (FCC)

OmyaPharm in collaboration with Omya International AG, Oftringen
FCC is pharmacologically inert, highly porous material with unique properties such as a large specific surface area and high porosity. FCC features unique lamellar structure, which is responsible for superior compatibility and compressibility properties. Compounding of FCC with other materials enables fast and efficient industrialization of complex formulations such as Orally and Fast Disintegrating Tablets and Minitablets, oral delivery of bioproteins, geometry-controlled drug release, gastroretentive floating dosage forms, mucoadhesive drug-loaded microparticles for colon drug delivery and local therapy, pediatric formulations and formulations with enhanced patient compliance, and many others (see Roth et al, 2017).
Mucoadhesive drug-loaded microparticles for colon drug delivery and local therapy
This research aims to develop an oral drug delivery system for targeted and sustained colonic release to facilitate the treatment of colonic diseases, such as ulcerative colitis, Crohn’s disease, or colon cancer. The oral dosage form is designed to remain intact during its passage through the stomach and small intestine, with release in the large intestine, directly at the site of intended action. The project is a collaboration between the division of pharmaceutical technology and Tillotts Pharma and has been supported by a grant from the Swiss Commission for Technology and Innovation (CTI) (see Preisig et al, 2016).
Floating Gastric-Retention Formulations
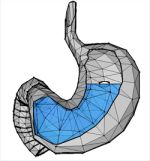
Functionalized calcium carbonate (FCC) can be successfully used to design floating gastric-retention formulations. FCC has an inherent low density and can therefore be used in an oral dosage form that floats in the stomach contents to achieve gastric retention and prolonged release rates of drugs in the upper part of the gastrointestinal tract (see Thesis Eberle V, 2016).
Orally and Fast Disintegrating Tablets and Minitablets
Application of FCC enables development of novel and promising drug delivery systems, such as orally disintegrating tablets (ODT). FCC-based oral dosage forms can be designed for short disintegration time preserving excellent mechanical strength. Use of FCC derivative products allows reduction in unit operations and conversion to direct compression manufacturing approach (see Wagner-Hattler et al, 2017).
Pediatric formulations and formulations with enhanced patient compliance
In collaboration with UKBB
Orally disintegrating tablets (ODT) are comfortable and safe drug delivery methods, beneficial for all age groups of patients. In this research project we investigate the perception of the orally dispersible tablet (ODT) based on FCC, varying approaches for mouthfeel enhancement and manufacturing settings. Using a visual analog scale (VAS) the roughness in the mouth, good taste, pleasant feeling, urge to drink, etc., can be assessed.
Geometry-controlled drug release
This research project is an example of development of an FCC derivative material, a new mineral-polymer composite (FCC-PCL). Such materials allow usage of complex geometries to aid in development of controlled release tablet formulations (Tablet-In-Cup, TIC). A comparative analysis suggests efficient formation of a complex, stable and impermeable geometries for constrained drug release modifications under compression. The TIC device produced with this new material has high physical stability, good binding properties and good compactibility due to enhanced plasticity of the composite material, which was not present in the individual components (see Wagner-Hattler et al, 2017).
Oral delivery of bioproteins
FCC is a porous microparticulate material with a nano-structured, lamellar surface, which is very promising in the field of oral drug delivery. In this research direction we use FCC as carrier for biomolecules e.g. lysozyme and bovine serum albumin in order to investigate its suitability to deliver protein based drugs. We could demonstrate a high loading efficiency of model proteins (>90%), while preserving enzyme activity. Our results suggest that FCC is a suitable pharmaceutical excipient for delivery of proteins.
Abuse-deterrent Formulations
To address patient safety and to restrict any misuse of medical products used in opioid substitution therapy we propose a novel approach to manufacture mechanical barrier-based formulations. This solid oral pharmaceutical dosage form provides extended release of active ingredients using polycaprolactone (PCL) as an enforcement and release rate-controlling component (Patent, Patent)
Cellular Automata-Based Computer Simulations of Drug-Release from Solid Formulations
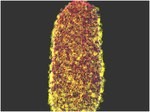
This research aims to further develop 3D cellular automata-based computation models to simulate physico-chemical processes taking place during tablet dissolution at the level of individual granules/crystals. Such dissolution models are highly computation-intensive; hence the use of specialized parallel-computing hardware is necessary. Currently, our software models are designed to work on General Purpose Graphic Processing Units (NVIDIA GPGPUs) and are programmed to take advantage of true computation parallelism (see Eberle et al, 2015).
Combination Drug Formulations for Diagnostic Purposes “CombiCap”

Collaboration with the Division of Clinical Pharmacology and Toxicology
Combination drugs with more than 4 active substances are extremely rare, yet very beneficial for oral administration of “drug cocktails”, which are often used as a diagnostic tool for metabolic phenotyping. Current research proposes a drug formulation combining 6 drugs made as minitablets, which helps to overcome a fixed drugs ratio problem, which is a known drawback of combination medicines. This project is a collaboration between the Division of Pharmaceutical Technology and the Division of Clinical Pharmacology of the University Hospital Basel, to promote the «Basel Cocktail» phenotyping approach (see Camblin et al, 2016)
Formulation by hot-melt extrusion
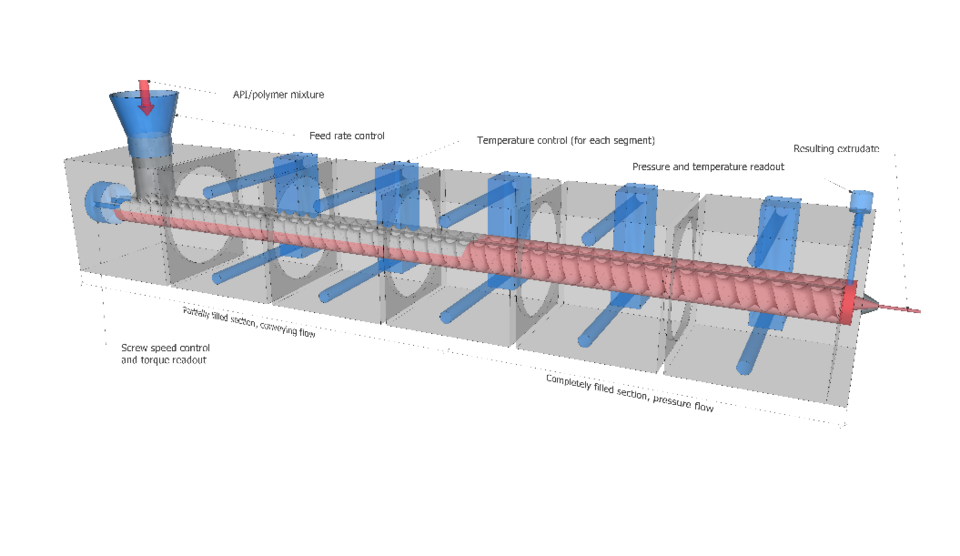
Manufacturing amorphous solid dispersions by hot-melt extrusion is a promising method to increase the oral bioavailability of low soluble drugs. This is a step towards better understanding of the process of hot-melt extrusion and application of resulting formulations in drug delivery. On the process level, we propose a mechanistic model linking process parameters to product quality. On the application level, we investigate the in vitro and in vivo properties of resulting formulations and their particulate carriers forming in situ. Ultimately, we aim for proof in human of a new formulation in a clinical setting.
Development of anthelmintic formulations with Oxantel Pamoate and Moxidectin for treatments in Africa, Tanzania
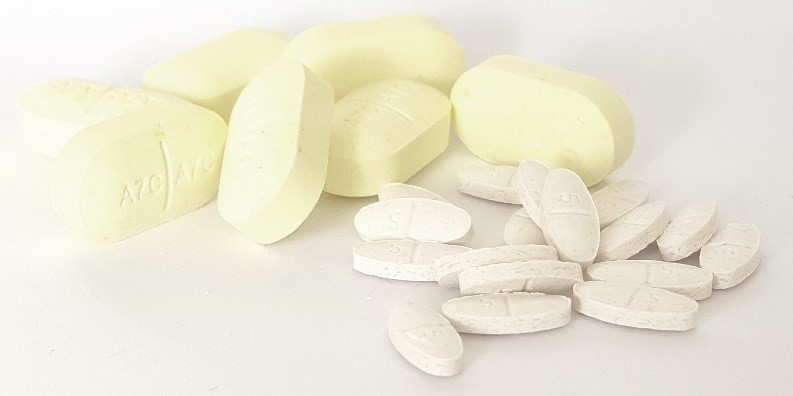
In Collaboration with Swiss Tropical and Public Health Institute (Swiss TPH)
In collaboration with the Swiss TPH our laboratories develop and manufacture tablets for treatment of hookworm infections in endemic regions such as Africa, Tanzania and Asia (see Moser et al, 2017).
