Engineered Nanomaterials for Drug Targeting
Different Delivery Systems - Liposomes, Polymer NPs, Inorganic NPs
Cancer therapy is a very broad medical field with various different treatment approaches. One possibility is the usage of nanoparticles. They are able to kill tumor cells either directly (e.g. via oxidative stress or DNA damage), or they act as carriers for anti-tumor agents. Using such a nanocarrier approach enables therapeutic agents to permeate efficiently and specifically into tumor tissue via passive or active targeting resulting in reduced side effects in normal tissue.
A wide range of nanomaterials based on organic, inorganic, lipid, protein, or glycan compounds as well as on synthetic polymers have been employed for the development of new cancer therapeutics (Kettiger et al. 2013; Wicki et al. 2015).
Physico-chemical characterization
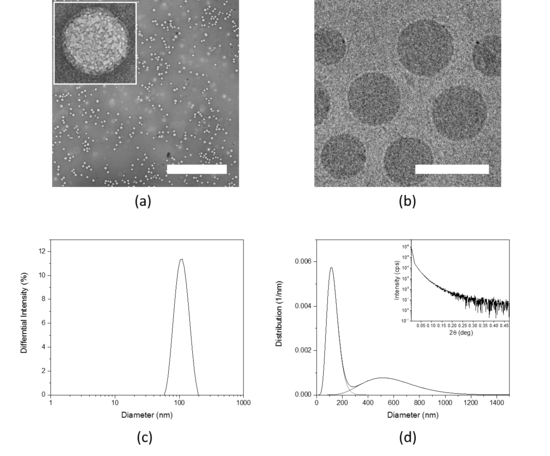
Physico-chemical properties of new emerging nanomaterials and nanoparticulate drug delivery systems have a strong influence on their interaction with biological matter, their bioavailability and biodistribution. Therefore, the physico-chemical characteristic of nanomaterials need to be precisely defined.
We perform thorough physico-chemical characterization of the nanomaterials developed in our group and we use different techniques to determine their properties such as size, shape, surface properties, chemical modifications and more.
Nanotoxicology in Drug Discovery
Nanotoxicology is the study of the adverse effects of engineered nanomaterials (ENMs) on living organisms and the ecosystems, including the prevention and amelioration of such adverse effects.
Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology; G. Oberdörster; J Internal Medicine; (2010)
Although nanoparticles bare an undisputable potential in the health and life science sectors, these formulations also challenge our current knowledge and strategies when it comes to guaranteeing their safety. It is known that particles in the nano-range may inherit unique properties and (therefore) exert different adverse effects than their bulk material. Such discrepancies demand for alternative toxicological assessments and new safety guidelines for these materials.
In collaboration with the Swiss Center for Applied Human Toxicology (SCAHT) and the EU founded NanoReg2 consortium, we focus our research on nanoparticle-mediated acute toxic effects in vitro and in vivo ranging from simple viability studies to signaling pathway analysis. With this knowledge, we aim at establishing characterization strategies and assay toolboxes that provide robust information on how nanoparticles interact with organisms (see Kettiger et al, 2015; Siegrist et al, 2017). These insights should ultimately help authorities to establish much needed nanosafety guidelines.
Heptocyte Specific Drug Targeting

Hepatic disorders affect millions of people around the globe and incidence rates are further increasing. While survival rates have improved for most diseases during recent decades, liver diseases still represent a considerable public health burden. Current therapies for diseases of hepatocytes are limited and in most cases only treat symptoms. Therefore, improved therapeutic technologies are urgently needed.
In Vitro / In Vivo Properties
Nanoparticles can improve the therapeutic performance of drugs by e.g. improving their pharmacokinetic properties and enhance the uptake by target cells. The understanding of interactions between nanoparticles and cells, as well as living organisms, is therefore a pivotal step in the development of nanomedicines.
Zebrafish

Nanomedicines have gained much attention for the delivery of small molecules or nucleic acids as treatment options for many diseases. However, the transfer from experimental systems to in vivo applications remains a challenge since it is difficult to assess their circulation behavior (pharmacokinetics) in the body at an early stage of drug discovery. Thus, innovative and improved concepts are urgently needed to overcome this issue and to close the gap between empiric nanoparticle design, in vitro assessment, and first in vivo experiments using rodent animal models. Therefore, we are validating the zebrafish as an early and easy accessible in vivo tool for the optimization of nanoparticulate drug delivery systems. For this purpose, we are using different transgenic fluorescent zebrafish lines and confocal microscopy (see Sieber et al, 2017).
Gene therapy
Gene therapy is one of the most rapidly growing areas in nanomedicine research. The delivery of foreign nucleic acids, e.g. siRNA, mRNA or DNA, into human cells offers huge opportunities for biomedical applications. Depending on the site of action of the nucleic acids, they may require escape from endosomes and delivery to the cytosol or nucleus. Gene delivery systems can be used to exert a therapeutic effect (i.e. introduction of a functional gene into cells suffering from a damaged gene), to modulate cellular functions (i.e. protein knockdown using siRNA), for monitoring purposes (i.e. using green fluorescent protein, GFP).

